Describe the Relationship Between Periodic Table Group and Valence Electrons
The periodic table and valence electrons worksheet answers. 3 valence electrons ex.

Lesson 3 Bonding The Periodic Table Diagram Quizlet
Valence Electrons Ions and the Periodic Table.
. For atoms with 8 valence electrons there is no change. The periodic table is arranged in order of increasing atomic numbers. Valence electrons determine the chemical properties and reactivity of an element.
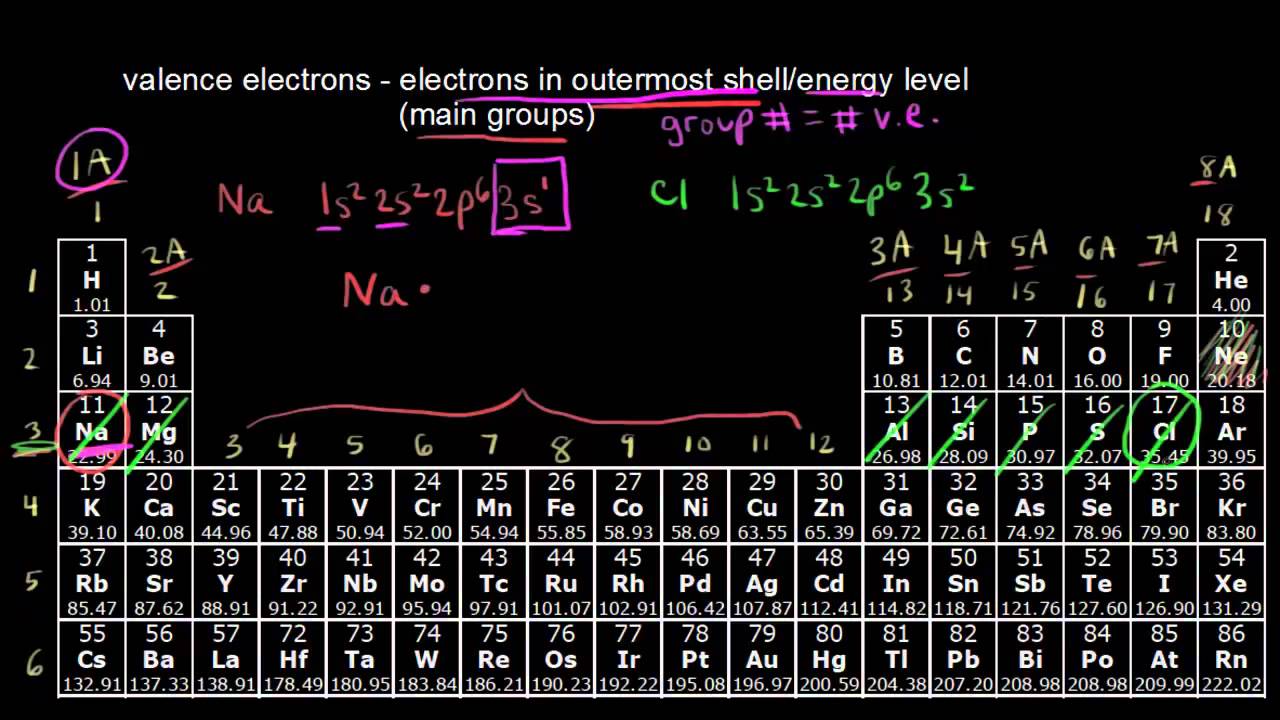
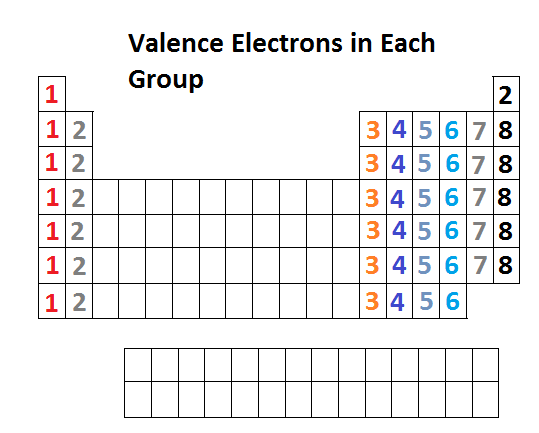
Valence electrons relate to the position of elements within the groups and periods of the periodic table and also their position within blocks. The position of an atom in the periodic table represents the number of valence electrons present in the outermost shell of the atom. The number of valence electrons from top to bottom of the group on the periodic table remains thesame because elements in the same group has thesame number of valence electrons in their outermost shell.
Elements are usually given temporary names at this point and are given their permanent names aCC0rding to international guidelinesCC0Max Pixel MORE FROM QUESTIONSANSWEREDNET Score. Locate and name the four blocks of the periodic tableExplain the reasons for these names. Because elements within a column in the periodic table have the same number of valence electrons they also have similar chemical properties.
Carbon represented as C is in Group 4A and has 4 valence electrons. Overall substances are made of up atoms. Ca has two valence electrons.
Solution for Describe the relationship between the properties of an element and the number of valence electrons that it contains. Describe the relationship between an elements row number in the periodic table and the highest principal quantum number in the elements electron configuration. Atoms which are found in the same group have the same number of valence electrons.
Elements in group 1A have only one valence electron and each group A column to the right adds one more valence electron. The number of valence electrons an atom has determines its location in the period. The electrons in the outermost or valence shell are especially important because they can engage in the sharing and exchange that is.
For main group elements the group number is also the number of valence electrons. First week only 499. All elements in group 17 react similarly because they all possess 5 valence electrons.
Outer shell electrons are also known as valence electrons. Across each row or period of the periodic table the number of valence electrons in groups 1 2 and 13 18 increases by one from one element to the next. Describe the locations in the periodic table and the.
Valence electrons are the outermost electrons of an atom. The number of valence electrons from left to right across the period increases across the period. The names of groups and periods on the periodic chart are alkali metals alkaline earth metals transition metals halogens and noble gases.
Valence electrons are the electrons present in the outermost shell of an atom. They are located between metals and nonmetals on the periodic table. 2 valence electrons ex.
Describe the relationship between the number of valence electrons in the elements of the periodic table and the groups and periods in the table. Metalloids have properties of metals and nonmetals. The properties of an atom relate directly to the number of electrons in various orbitals and the periodic table is much like a road map among those orbitals such that chemical properties can be deduced by the position of an element on the table.
The chemical properties of elements are largely determined by the number of valence electrons they contain. The first 20 elements of the periodic table are. Atoms in Groups 13 and 18 have 3 and 8 valence electrons respectively.
Start your trial now. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. Discuss the relationship between group configurations and group numbers.
Core electrons determine how atoms interact with each other. For example atoms in Groups 1 and 2 have 1 and 2 valence electrons respectively. The number of positive charges in the nucleus determines how many electrons normally surround the nucleus.
Valence Electrons and Periodic Table. For purposes of describing chemical behavior an atom can be considered as a positively charged nucleus surrounded by negatively charged electrons orbiting in concentric spherical shells. The periodic table of elements is a table is a method of classification of elements that arranges the elements with similar properties together in groups.
Explain the relationship between electrons in sublevels and the length of each period of the periodic table. Describe the relationship between an elements row. Conveniently the Group Number at the top of each column in the periodic table also gives the number of valence electrons.
For atoms with 4 valence electrons it can go either way. The Relationship of Electrons and Valence. For example Boron represented as B in the periodic table is in Group 3A and has 3 valence electrons.
The columns that were set up to group elements by similar chemical properties turn out to be the exact same columns defined by the number of valence electrons. Elements that contain1 valence electron falls under Group 12 valence electrons falls under Group 23 valence electrons falls under. Their properties are periodic because the number of valence electrons is periodic.
How does this relation- ship differ for main-group elements transition elements and inner transition elements. This is a tabular arrangement of the chemical elements ordered by their atomic number electron configuration and recurring chemical properties whose structure shows periodic trends. Every atom is created of a nucleus containing protons and neutrons enclosed by electrons.
The element with the electron configuration of Ne3s2 3p4 has six valence electrons so within its period of 3 it must be a group 6. For example Beryllium Magnesium and Calcium are in group 2 and will therefore have 2 valence electrons. This is the official periodic table given by Cambridge Periods show the number of electrons in an element The group number is the number of electrons in their valence shell The eight groups have elements.
Groups in the periodic table. Describe the relationship between periodic table group and valence electrons worksheet. 1 valence electron ex.
Up to 24 cash back 52 Objectives. Elements are grouped according to the number of electrons they have in their outer shell. Based on a StudySmarter Original by Olive Odagbu.
Carbon is in Group 4 4 valance electrons Put one on each side.
What Is The Number Of Groups And Period Of An Element That Has Three Shells And Three Electrons In A Valence Shell Quora

Counting Valence Electrons For Main Group Elements Video Khan Academy
No comments for "Describe the Relationship Between Periodic Table Group and Valence Electrons"
Post a Comment